Anti- Cytomegalovirus antibodies, TNF- α, IL-19, CD4, CD163, and VEGF-A serum levels in Patients with Breast Cancer
Anti- Cytomegalovirus antibodies, TNF- α, IL-19, CD4, CD163, and VEGF-A serum levels in Patients with Breast Cancer
DOI:
https://doi.org/10.63841/iue24500Keywords:
Cytomegalovirus, Breast cancer TNF-a, CD4, CD 163, IL-19Abstract
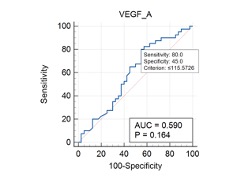
Breast cancer is the most prevalent malignant tumor detected in women globally. Cytomegalovirus (CMV) infection has been linked to immune system modulation, potentially altering cytokine levels and establishing a tumor-promoting microenvironment. The aim of the study was to determine the effect of CMV infection and cytokine levels in breast cancer progression. A total of 80 blood samples were analyzed, comprising 40 samples from breast cancer patients admitted to Nanakaly Hospital's / oncology department and 40 samples from healthy controls selected randomly. CMV-IgG and IgM levels were tested using a Cobas analyzer to determine seropositivity among the participants in the study. ELISA technique was used for determining serum cytokine levels including interleukin-19 (IL-19), tumor necrosis factor-alpha (TNF-α), vascular endothelial growth factor (VEGF), and soluble CD163 and CD4. Statistical analysis was used to determine the associations between CMV seropositivity, cytokine levels, and breast cancer progression. TNF-α emerged as the most diagnostically accurate biomarker due it is high area under curve (AUC). It demonstrated statistically significant odds ratios as a risk factor for breast cancer development (P= 0.048). CD4, IL-19, exhibited minimal impact on breast cancer development (p=0.659 and p=0.564 respectively), indicating weak associations. Vascular endothelial growth factor A (VEGF A) did not show a significant association with the development of the breast cancer. Similarly, CD 163 showed no relationship with breast cancer progression. In addition, in this study the serum concentrations of all immunological and tumor markers were elevated in CMV IgG positive controls as detected in breast cancer patients. It is concluded that high levels of TNF-α in both CMV infected and breast cancer patients indicates a positive correlation between the progression of breast cancer and CMV infection. Slight increase in the other estimated markers may also confirm this correlation in this study.
Downloads
References
W. F. Anderson, P. S. Rosenberg, A. Prat, C. M. Perou, and M. E. Sherman, “How many etiological subtypes of breast cancer: two, three, four, or more?,” Journal of the National Cancer Institute, vol. 106, no. dju165, 2014.
R. L. Siegel, K. D. Miller, and A. Jemal, “Cancer statistics, 2019,” CA: A Cancer Journal for Clinicians, vol. 69, pp. 7–34, 2019.
J. B. Swann and M. J. Smyth, “Immune surveillance of tumors,” The Journal of Clinical Investigation, vol. 117, pp. 1137–1146, 2007.
L. E. Harkins, L. A. Matlaf, L. Soroceanu, K. Klemm, W. J. Britt, W. Wang, K. I. Bland, and C. S. Cobbs, “Detection of human cytomegalovirus in normal and neoplastic breast epithelium,” Herpesviridae, vol. 1, pp. 1–10, 2010.
Z. Yang, X. Tang, M. E. Hasing, X. Pang, S. Ghosh, T. P. McMullen, D. N. Brindley, and D. G. Hemmings, “Human cytomegalovirus seropositivity and viral DNA in breast tumors are associated with poor patient prognosis,” Cancers, vol. 14, no. 1148, 2022.
N. E. Mangare, M. W. Muturi, and G. Gachara, “Seroprevalence of cytomegalovirus infection and associated risk factors among human immunodeficiency virus infected patients attending Thika Level 5 Hospital, Kenya,” Open Journal of Immunology, vol. 8, pp. 1–8, 2018.
H.-C. Shen, J.-Y. Feng, C.-Y. Sun, J.-R. Huang, Y.-M. Chen, W.-C. Chen, and K.-Y. Yang, “Analysis of the effect of cytomegalovirus infection in clinical outcomes and prolonged duration of SARS-CoV-2 shedding in intensive care unit patients with COVID-19 pneumonia,” Therapeutic Advances in Respiratory Disease, vol. 17, no. 17534666231209150, 2023.
D. Ziehe, A. Wolf, T. Rahmel, H. Nowak, H. Haberl, L. Bergmann, K. Rump, B. Dyck, L. Palmowski, and B. Marko, “Exploring the relationship between HCMV serostatus and outcomes in COVID-19 sepsis,” Frontiers in Immunology, vol. 15, no. 1386586, 2024.
L. Soroceanu, L. Matlaf, S. Khan, A. Akhavan, E. Singer, V. Bezrookove, S. Decker, S. Ghanny, P. Hadaczek, and H. Bengtsson, “Cytomegalovirus immediate-early proteins promote stemness properties in glioblastoma,” Cancer Research, vol. 75, pp. 3065–3076, 2015.
M. Michaelis, H. W. Doerr, and J. Cinatl Jr., “The story of human cytomegalovirus and cancer: increasing evidence and open questions,” Neoplasia, vol. 11, pp. 1–9, 2009.
S. Haidar Ahmad, R. El Baba, and G. Herbein, “Polyploid giant cancer cells, cytokines and cytomegalovirus in breast cancer progression,” Cancer Cell International, vol. 23, no. 119, 2023.
S. Commins, J. W. Steinke, and L. Borish, “The extended IL-10 superfamily: IL-10, IL-19, IL-20, IL-22, IL-24, IL-26, IL-28, and IL-29,” Journal of Allergy and Clinical Immunology, vol. 121, pp. 1108–1111, 2008.
L. Dumoutier, C. Leemans, D. Lejeune, S. V. Kotenko, and J.-C. Renauld, “Cutting edge: STAT activation by IL-19, IL-20 and MDA-7 through IL-20 receptor complexes of two types,” The Journal of Immunology, vol. 167, pp. 3545–3549, 2001.
N. Jan, H. Qayoom, M. Alkhanani, A. Almilaibary, and M. A. Mir, “Elucidation of interleukin-19 as a therapeutic target for breast cancer by computational analysis and experimental validation,” Saudi Journal of Biological Sciences, vol. 30, no. 103774, 2023.
C.-H. Hsing, H.-C. Cheng, Y.-H. Hsu, C.-H. Chan, C.-H. Yeh, C.-F. Li, and M.-S. Chang, “Upregulated IL-19 in breast cancer promotes tumor progression and affects clinical outcome,” Clinical Cancer Research, vol. 18, pp. 713–725, 2012.
I. Shabo, K. Midtbö, H. Andersson, E. Åkerlund, H. Olsson, P. Wegman, C. Gunnarsson, and A. Lindström, “Macrophage traits in cancer cells are induced by macrophage-cancer cell fusion and cannot be explained by cellular interaction,” BMC Cancer, vol. 15, pp. 1–11, 2015.
S. A. Abou Shousha, B. Hussein, Y. Shahine, G. Fadali, M. Zohir, Y. Hamed, M. Hemedah, S. A. Baheeg, A. Ibrahim, and M. El Shannawy, “Angiogenic activities of interleukin-8, vascular endothelial growth factor and matrix metalloproteinase-9 in breast cancer,” Egypt J Immunol, vol. 29, pp. 54–63, 2022.
P. Łaskowski, B. Klim, K. Ostrowski, M. Szkudlarek, E. Litwiejko-Pietryńczak, K. Kitlas, A. Nienartowicz, and J. Dzięcioł, “Local inflammatory response in colorectal cancer,” Polish Journal of Pathology, vol. 67, pp. 163–171, 2016.
M. Dehghani, S. Sharifpour, Z. Amirghofran, and H. R. Zare, “Prognostic significance of T cell subsets in peripheral blood of B cell non-Hodgkin’s lymphoma patients,” Medical Oncology, vol. 29, pp. 2364–2371, 2012.
S. A. Quezada, K. S. Peggs, T. R. Simpson, and J. P. Allison, “Shifting the equilibrium in cancer immunoediting: from tumor tolerance to eradication,” Immunological Reviews, vol. 241, pp. 104–118, 2011.
M. F. Mercogliano, S. Bruni, P. V. Elizalde, and R. Schillaci, “Tumor necrosis factor α blockade: an opportunity to tackle breast cancer,” Frontiers in Oncology, vol. 10, no. 584, 2020.
A. Richardson, B. Cox, M. McCredie, G. Dite, J. Chang, D. Gertig, M. Southey, G. Giles, and J. Hopper, “Cytomegalovirus, Epstein–Barr virus and risk of breast cancer before age 40 years: a case–control study,” British Journal of Cancer, vol. 90, pp. 2149–2152, 2004.
R. M. Locksley, N. Killeen, and M. J. Lenardo, “The TNF and TNF receptor superfamilies: integrating mammalian biology,” Cell, vol. 104, pp. 487–501, 2001.
D. J. MacEwan, “TNF receptor subtype signalling: differences and cellular consequences,” Cellular Signalling, vol. 14, pp. 477–492, 2002.
F. Balkwill, “Tumor necrosis factor or tumor promoting factor?,” Cytokine & Growth Factor Reviews, vol. 13, pp. 135–141, 2002.
E. Papadopoulou, G. Tripsianis, K. Anagnostopoulos, I. Tentes, S. Kakolyris, G. Galazios, E. Sivridis, K. Simopoulos, and A. Kortsaris, “Significance of serum tumor necrosis factor-alpha and its combination with HER-2 codon 655 polymorphism in the diagnosis and prognosis of breast cancer,” The International Journal of Biological Markers, vol. 25, pp. 126–135, 2010.
J. Busselaar, S. Tian, H. van Eenennaam, and J. Borst, “Helpless priming sends CD8(+) T cells on the road to exhaustion,” Frontiers in Immunology, vol. 11, no. 592569, 2020.
R. E. Tay, E. K. Richardson, and H. C. Toh, “Revisiting the role of CD4+ T cells in cancer immunotherapy—new insights into old paradigms,” Cancer Gene Therapy, vol. 28, pp. 5–17, 2021.
Y.-Y. Chen, C.-F. Li, C.-H. Yeh, M.-S. Chang, and C.-H. Hsing, “Interleukin‐19 in breast cancer,” Journal of Immunology Research, vol. 2013, no. 294320, 2013.
T. O. Jensen, H. Schmidt, H. J. Møller, M. Høyer, M. B. Maniecki, P. Sjoegren, I. J. Christensen, and T. Steiniche, “Macrophage markers in serum and tumor have prognostic impact in American Joint Committee on Cancer stage I/II melanoma,” Journal of Clinical Oncology, vol. 27, pp. 3330–3337, 2009.
M. K. Tuck, D. W. Chan, D. Chia, A. K. Godwin, W. E. Grizzle, K. E. Krueger, W. Rom, M. Sanda, L. Sorbara, S. Stass, and W. Wang, “Standard operating procedures for serum and plasma collection: early detection research network consensus statement standard operating procedure integration working group,” Journal of Proteome Research, vol. 8, no. 1, pp. 113–117, 2009.
Roche Diagnostics, Elecsys® CMV IgG and IgM Package Insert, Mannheim, Germany: Roche Diagnostics, n.d.
A. Chiereghin, C. Pavia, L. Gabrielli, G. Piccirilli, D. Squarzoni, G. Turello, D. Gibertoni, G. Simonazzi, M. G. Capretti, M. Lanari, and T. Lazzarotto, “Clinical evaluation of the new Roche platform of serological and molecular cytomegalovirus-specific assays in the diagnosis and prognosis of congenital cytomegalovirus infection,” Journal of Virological Methods, vol. 248, pp. 250–254, 2017.
Downloads
Published
Issue
Section
License
Copyright (c) 2025 Academic Journal of International University of Erbil

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.